Rapid Test
Rapid, point-of-care tests make it easier for people to access testing and ensure that test results are received and are acted upon immediately.
Rapid, point-of-care tests make it easier for people to access testing and ensure that test results are received and are acted upon immediately. When people receive their test results immediately, they can more quickly access to treatment and care. Rapid tests also give a good opportunity for pre-test and post-test counseling. People who seek seeking testing and counseling voluntarily are also often the ready for behavior change and messaging about prevention and accessing treatment can be effective.
It is recommended that health-care providers familiarize themselves with the performance characteristics of the type of test used as these inform use and counselling. Health-care providers should be aware that rapid HIV / HCV tests are less sensitive than laboratory-based tests and may therefore give false negative results in the early state of infection. Reduced sensitivity has also been reported in advanced disease/AIDS. In addition, as with all tests, the positive predictive value of a reactive test is reduced in low prevalence settings meaning that false positive results will occur to a different extent depending on the setting and population undergoing screening.
Point of care tests that use sample types other than blood, such as plaque, may be subject to more variation in assay performance and sensitivity. Obtaining a blood sample for laboratory testing is recommended in all patients with reactive or indeterminate results and in patients with a negative test if recent infection is suspected. Sites using rapid tests should be overseen by the local laboratory and have a robust quality assurance programme.
WHAT DOES A RAPID TEST MEAN?
- Test is rapid to perform : 1-30 min.
- The test do not require specialized equipment
- The tests are easy to perform
- Some tests can be done from finger prick blood or saliva
- Rapid tests do not find infections more rapidly than laboratory tests
Most rapid tests detect both HIV-1 and HIV-2, but most of these tests do not differentiate between them.
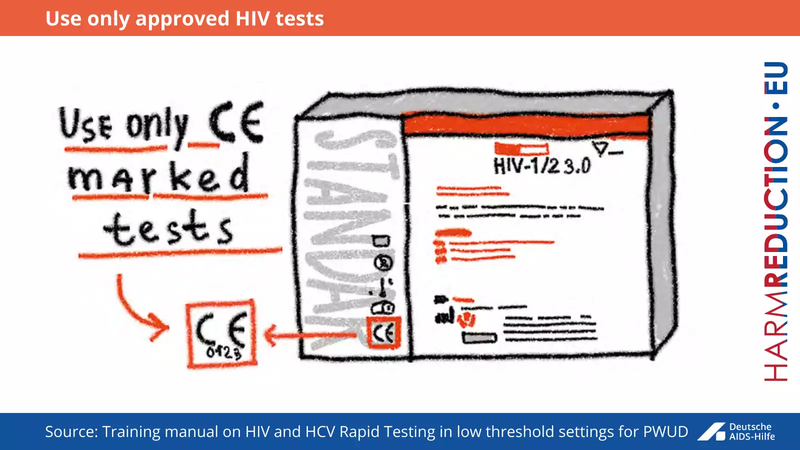
Only tests with a CE marking can be used for testing. The CE marking is a certification mark that indicates conformity with health, safety, and environmental protection standards for products sold within the European Economic Area (EEA). It is the manufacturer’s declaration that the product meets the requirements of the applicable CE directive. This means that performance of the test has been evaluated by the appropriate Notified Body. The approved CE mark consists CE logo and the four digit identification number (below the CE logo) of the Notified Body involved in the conformity assessment procedure. If the test is not officially CE marked and properly evaluated, the test's performance is not known.
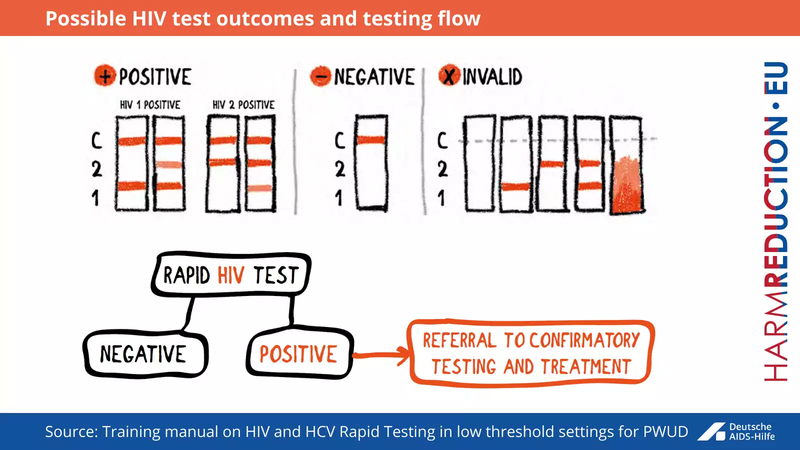
The test should be performed strictly according to the manufacturer’s instructions. There are three possible outcomes for rapid antibody tests (see Fig below).
- The result is positive when the reaction line is shown at the control and patient window
- The result is negative when the reaction line is shown only at the control window
- No result at all when there is no reaction line at the control window